
Total Clean Utility Solutions for the Life Sciences Industry
Engineering, compliance, and execution for high-purity utility systems delivered with precision and reliability.
Full Regulatory Compliance
USP, GMP, ASME BPE
End-to-End Solutions
From design to installation and validation
Proven Industry Experience
+16 years of delivering solutions for life sciences
Trusted Technology Partners:
Dockweiler, Samson SED, Orbitalum, SterileX
Engineering Solution

Purified Water Systems
Design and build RO-EDI systems fully compliant with USP and GMP standards.

CIP & SIP Systems
Automated CIP skids for cleaning tanks, pipes, and process equipment with precision and reduced resource use.

Mixing & Transfer Systems
Custom-engineered solutions for blending, dosing, and transferring pharmaceutical-grade liquids.
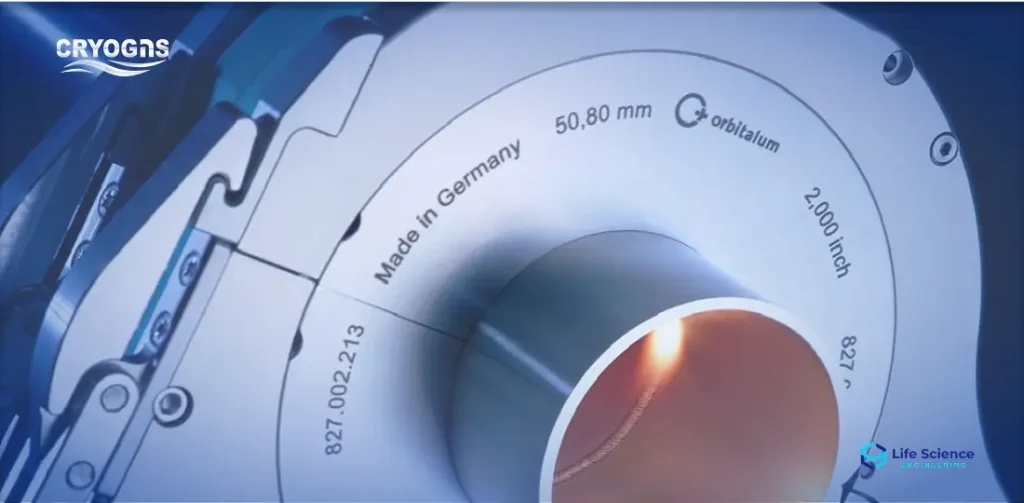
Orbital Welding System
Clean orbital welding services with documentation, borescope inspection, and ASME BPE compliance.
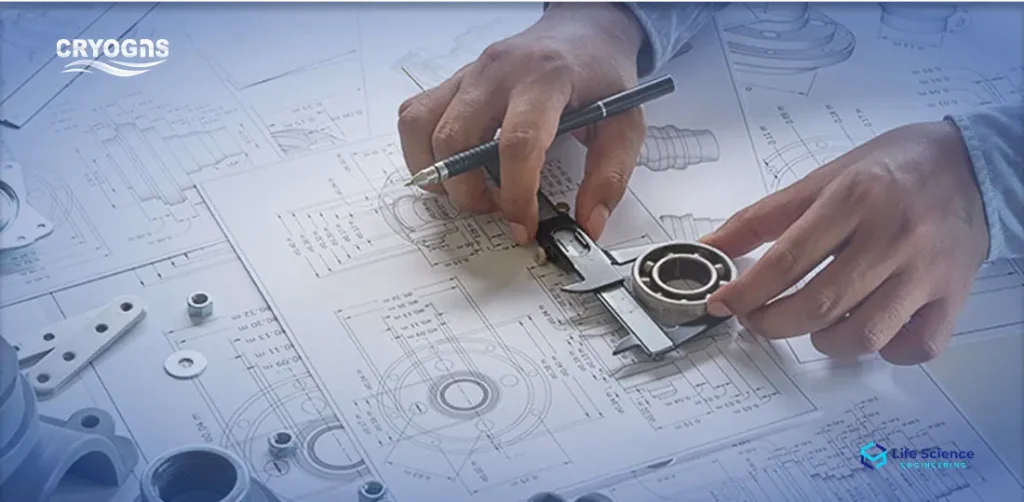
Clean Utility Integration
Design and install WFI, Pure Steam, and Clean Compressed Air systems with full validation support.
Compliance & Validation Methodology
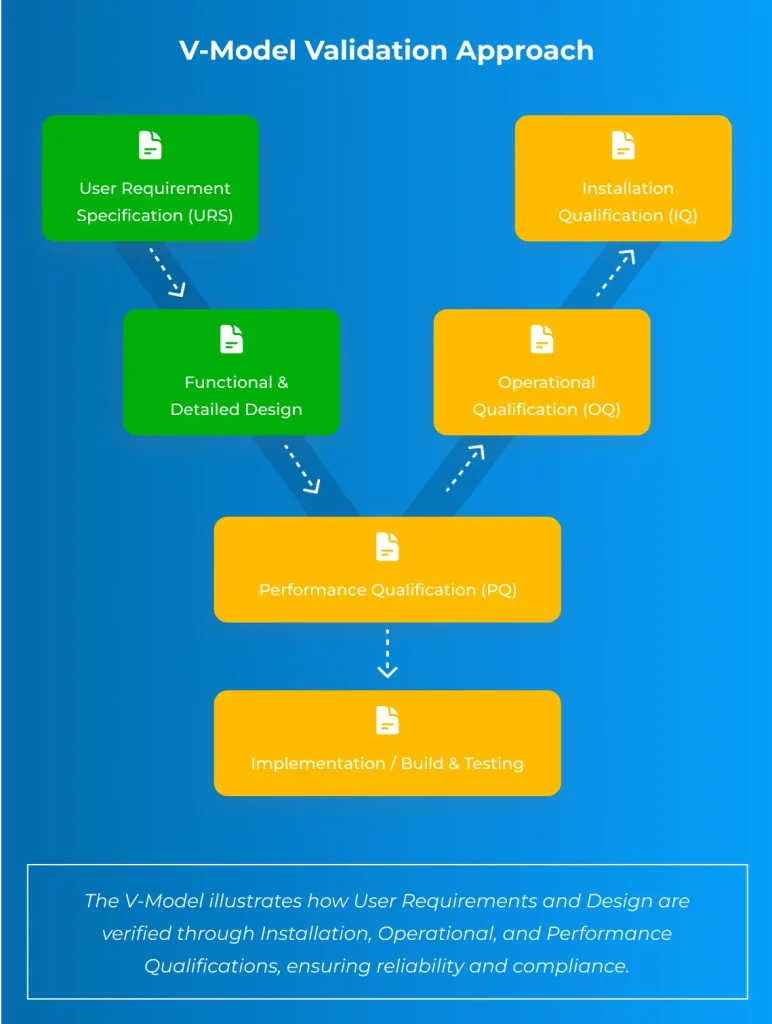
V-Model Validation Approach
We follow the internationally recognized V-Model lifecycle in all our pharmaceutical engineering projects, ensuring compliance and traceability from design to qualification.
User Requirement Specification (URS)
Define customer needs & compliance.
Design Qualification (DQ)
Ensure design meets GMP & project.
Functional & Detailed Design
Specify automation, control & system.
Factory Acceptance Test (FAT) & Site Acceptance Test (SAT)
verifying functionality prior to installation and on-site.
Installation Qualification (IQ)
Document proper equipment installation.
Operational Qualification (OQ)
Test functions under normal conditions.
Performance Qualification (PQ)
Confirm reliable performance in production.
The V-Model ensures traceability, documented evidence, and compliance with USP, GMP, ASME BPE, and GAMP 5, making it a trusted framework for pharmaceutical system validation.
Risk-Based Approach (ICH Q9)
We apply a Risk-Based Approach (ICH Q9) to identify, control, and review CQA and CPP across the lifecycle.
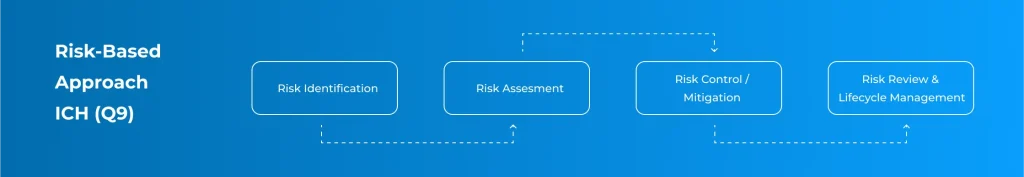
Ensures decisions prioritize quality, safety, compliance, and efficiency.
Risk Assessment
Identifying critical quality attributes (CQA) and critical process parameters (CPP).
Risk Mitigation
Applying appropriate controls, redundancy, and monitoring for high-risk elements.
Documentation
Ensuring that critical decisions are traceable and justified.
Continuous Review
Updating risk assessments during system changes or lifecycle extensions.
Industries Coverage
Pharmaceutical
Biotechnology
Food & Beverage
Nutraceuticals & Dietary Supplements
Cosmetic, Medical Devices & Healthcare

